
M. Samy El-Shall, Ph.D.
Professor,
Mary Eugenia Kapp Chair in Chemistry
mselshal@vcu.edu
(804) 828-3518
Oliver Hall 3041
Education
B. Sc. (Chemistry) with Distinction, Cairo University, Egypt, 1976
Ph.D. (Physical Chemistry) with Distinction, Georgetown University, 1985
Postdoctoral, University of California, Los Angeles (UCLA), 1986-1988
Honors and awards
- Virginia Outstanding Scientist 2018, Awarded by the Virginia Governor
- Mary Eugenia Kapp Endowed Chair in Chemistry, 2017, Virginia Commonwealth University
- University Award of Excellence, 2016, Virginia Commonwealth University
- American Association for the Advancement of Science (AAAS), Elected Fellow, 2013
- Jefferson Science Fellow (JSF), U.S. Department of State, 2012-13
- American Physical Society (APS), Elected Fellow, 2012
- University Distinguished Scholarship Award, VCU, 2011
- Distinguished Research Award, Virginia Section of the American Chemical Society, 2009
- SAE Innovative Research Award in Automotive Lubricants, Society of Automotive Engineering, 2009
- Jabir Ibn Hyyan Award for Advances in Nanomaterials & Nanotechnology, Saudi Chemical Society, 2007
- Outstanding Faculty Award, State Council of Higher Education of Virginia (SCHEV), 1999
- VCU Board of Visitors Teaching Fellow, 1998
- Distinguished Scholar Award, College of Humanities and Sciences, VCU, 1996
- Exxon Education Award, 1994 -1996
- The Zorbach Prize for the outstanding PhD thesis in Chemistry, Georgetown University, 1986
- Certificate of Merit, National Bureau of Standards (NBS, NIST), 1985
Research interests
- molecular clusters
- intracluster reactions
- gas phase and cluster polymerization
- nanostructured materials
- heterogeneous catalysis
- nanoalloys
- graphene and carbon nanotubes
- nucleation phenomena
- ion-induced nucleation
- nucleation on nanoparticles
Our research interests are in the general areas of molecular cluster ions, gas phase and intracluster ionic polymerization, nucleation phenomena, nanostructured materials and heterogeneous catalysis. The major goal is to gain insights as to how the properties of matter evolve as the size of a material system ranges from molecular to macroscopic dimensions.
Research underway
Formation of complex organics by gas phase and intracluster ion-molecule reactions
This area involves the application of physical chemistry/chemical physics methods and techniques such as supersonic beam expansion, molecular and metal-containing clusters, laser ionization, ion-molecule and intracluster reactions, ion mobility and time-of-flight mass spectrometry in addition to quantum chemistry calculations.
The research is focused on the formation mechanisms, structures and reactions of complex organics in the gas phase and within molecular clusters. These complex organics contain diverse molecules such as acetylene, hydrogen cyanide, benzene, and polycyclic aromatic hydrocarbons (PAHs). These molecules are formed in flames and combustion processes and they contribute to soot formation and the generation of organic aerosols which could affect human health and the environment. These molecules are also formed in interstellar clouds and solar nebula where they contribute to the observed complex organics in space.
The gas phase and cluster ion experiments are uniquely suited for the discovery of novel catalytic pathways that can lead to the formation of complex organics. These experiments identify the chains of reactions leading to the formation of PAHs found in soot, combustion, organic aerosols, interstellar clouds and meteorites. They also provide detailed information on the kinetics, growth mechanisms, energy barriers, structures, isomerization, and reactivity of large organic ions as well as their complexes with associated solvent molecules in different environments.
Selected publications (clusters and gas phase ion-molecule reactions)
Non-Covalent Interactions of Hydrogen Cyanide and Acetonitrile with the Quinoline Radical Cation via Ionic Hydrogen Bonding, K. A. Mason, A. C. Pearcy, K. U. Lao, Z. A. Christensen, M. S.
El-Shall, Chem. Phys. Letters 2020, 754, 137744.
Formation of the Oxonium Phenol Ion in the Stepwise Hydration of the Phenyl Cation in the Gas Phase S. Elroby, I. K. Attah, S. P. Platt, M. S. El-Shall, S. G. Aziz, R. Hilal, Journal of Molecular Liquids 2020, 322, 114541.
Structures of Benzonitrile Dimer Radical Cation and the Protonated Dimer: Observation of Hydronium Ion Core Solvated by Benzonitrile Molecules, K. A. Mason, A. C. Pearcy, A. M. Hamid, M. S. El-Shall, J. Chem. Phys. 2019, 150, 124303.
Ionic Hydrogen and Halogen Bonding in the Gas Phase Association of Acetonitrile and Acetone with Halogenated Benzene Cations, A. C. Pearcy, K. A. Mason, M. S. El-Shall, J. Phys. Chem. A 2019, 123, 1363-1371 (Journal Cover).
Nucleophilic Aromatic Addition in Ionizing Environments: Observation and Analysis of New C–N Valence Bonds in Complexes between Naphthalene Radical Cation and Pyridine, R. Peverati, S. P. Platt, I. K. Attah, S. Aziz, M. S. El-Shall, and M. Head-Gordon, J. Am. Chem. Soc. 2017, 139, 11923-11932.
Gas Phase Hydration of Halogenated Benzene Cations. Is it Hydrogen or Halogen Bonding?, K. A. Mason, A. C. Pearcy, I. K. Attah, S. P. Platt, S. G. Aziz, and M. S. El-Shall, Phys. Chem. Chem. Phys. 2017, 19, 18603-18611.
Observation of Covalent and Electrostatic Bonds in Nitrogen-Containing Polycyclic Ions Formed by Gas Phase Reactions of the Benzene Radical Cation with Pyrimidine, I. K Attah, A. R. Soliman, S. Platt, M. Meot-Ner, S. Aziz and M. S. El-Shall, Phys. Chem. Chem. Phys. 2017, 19, 6422-6432.
Unconventional CHδ+⋯ N Hydrogen Bonding Interactions in the Stepwise Solvation of the Naphthalene Radical Cation by Hydrogen Cyanide and Acetonitrile Molecules, S. P. Platt, I. K. Attah, M. S. El-Shall, R. Hilal, S. A. Elroby, S. G. Aziz, Phys. Chem. Chem. Phys. 2016, 18, 2580-2590.
Ion Mobility of the Radical Cation Dimers: (Naphthalene)2+. and Naphthalene+.(Benzene): Evidence for Stacked Sandwich and T-shape Structures, S. P. Platt, I. K. Attah, S. Aziz, and M. S. El-Shall, J. Chem. Phys. 2015, 142, 19112.
What is the Structure of the Naphthalene–Benzene Heterodimer Radical Cation? Binding Energy, Charge Delocalization, and Unexpected Charge-Transfer Interaction in Stacked Dimer and Trimer Radical Cations, I. K. Attah, S. P. Platt, M. Meot-Ner (Mautner), M. S. El-Shall, R. Peverati, and M. Head-Gordon, J. Phys. Chem. Lett. 2015, 6, 1111–1118.
Growth Kinetics and Formation Mechanisms of Complex Organics by Sequential Reactions of Acetylene with Ionized Aromatics, A. Soliman, I. K. Attah, A. M. Hamid, and M. S. El-Shall,
Inter. J. Mass Spectrom 2015, 377, 139-151.
Unconventional Hydrogen Bonding to Organic Ions in the Gas Phase. Stepwise Association of Hydrogen Cyanide with the Pyridine and Pyrimidine Radical Cations and Protonated Pyridine, A. M. Hamid, M. S. El-Shall, R. Hilal, S. Elroby and S. G. Aziz, J. Chem. Phys. 2014, 141, 054305, 1-10.
Formation of Covalently Bonded Polycyclic Hydrocarbon Ions by Intracluster Polymerization of Ionized Ethynylbenzene Clusters, P. O. Momoh, I. K. Attah, M. S. El-Shall, R. P. F. Kanters, J. M. Pinski, and S.A. Abrash, J. Phys. Chem. A 2014, 118, 8251-8263.
Proton-bound Dimers of Nitrogen Heterocyclic Molecules. Substituent Effects on the Structures and Binding Energies of Homodimers of Diazine, Triazine, and Fluoropyridine, I. K. Attah, S. P. Platt, M. Meot-Ner, S. G. Aziz, A. O. Alyoubi and M. S. El-Shall, J. Chem. Phys. 2014, 140, 114313, 1-11.
Formation of Nitrogen-Containing Polycyclic Cations by Gas-Phase and Intracluster Reactions of Acetylene with the Pyridinium and Pyrimidinium Ions, A. Soliman, A. M. Hamid, I. Attah, P. Momoh and M. S. El-Shall, J. Am. Chem. Soc. 2013, 135, 155-166.
Hydration of the Pyrimidine Radical Cation and Stepwise Solvation of Protonated Pyrimidine with Water, Methanol and Acetonitrile, A. M. Hamid, P. Sharma, R. Hilal, S. Elroby, S. G. Aziz, A. O. Alyoubi, and M. S. El-Shall, J. Chem. Phys. 2013, 139, 084304.
Substituent Effects on Non-Covalent Bonds: Complexes of Ionized Benzene Derivatives with Hydrogen Cyanide, I. K. Attah, A. M. Hamid, M. Meot-Ner, S. G. Aziz, A. O. Alyoubi and M. S. El-Shall, J. Phys. Chem. A 2013, 117, 10588-10579.
Stepwise Association of Hydrogen Cyanide and Acetonitrile with the Benzene Radical Cation: Structures and Binding Energies of (C6H6•+)(HCN)n, n = 1–6, and (C6H6•+)(CH3CN)n, n = 1–4, Clusters, A. M. Hamid, A. Soliman, and M. S. El-Shall, J. Phys. Chem. A 2013, 117, 1069-1078.
Nanostructured materials for energy applications and heterogeneous catalysis
Semiconductor nanocrystals


This area involves the development of new chemical and physical methods for the synthesis and characterization of size-and shape-controlled semiconductor and metal-semiconductor hybrid nanocrystals (NCs).
Our efforts have focused on the synthesis of nanostructures with well-defined geometrical shapes and their assembly as 2- and 3-D assemblies. Semiconductor nanocrystals (PbS, PbSe, CdSe, CdTe) of different sizes and shapes (dots, rods and tetrapods) are prepared using the microwave-assisted synthesis methods developed in our laboratory. We are also interested in the synthesis of metal-semiconductor hybrid nanostructures such as Au-ZnO and Au-CdSe of different shapes and sizes since they combine the tunable band gap of semiconductor quantum dots with the plasmonic effect of properties of these hybrid nanostructures, including absorption coefficient, band gap, and photocurrent for the development of efficient nanostructured photovoltaic devices. Nanostructured materials provide innovative strategies for designing next-generation energy conversion devices metallic nanoparticles. We optimize the optical and electrical properties of these hybrid nanostructures, including absorption coefficient, band gap, and photocurrent for the development of efficient nanostructured photovoltaic devices. Nanostructured materials provide innovative strategies for designing next-generation energy conversion devices.
Nanaocatalysis

Our research also involves the application of lasers for the synthesis of metal, metal oxide and alloy nanoparticles for applications as catalysts for a variety of chemical transformations such as low temperature CO oxidation, carbon-carbon cross-coupling reactions and Fischer-Tropsch synthesis of liquid hydrocarbons. The laser applications include the laser vaporization-controlled condensation (LVCC) and the laser vaporization-solvent capturing (LVSC) methods developed in our laboratory. These methods allow the synthesis of alloy nanoparticle catalysts with controlled composition and clean surfaces, and, in some cases, tunable catalyst-support interactions.
Graphene research
Recently, our laboratory has developed several methods for the synthesis of graphene and metal and semiconductor nanoparticles supported on graphene. Laser irradiation of graphene oxide (GO) in water results in deoxygenation of GO accompanied by strong photothermal energy conversion which can be used for the efficient conversion of solar energy into usable heat for a variety of thermal, thermochemical, and biomedical applications. Current research involves the coupling of the photothermal effects of GO with those from Au and other plasmonic nanostructures in order to develop highly efficient materials for the conversion of solar radiation into usable heat.
Heterogeneous catalysis on graphene
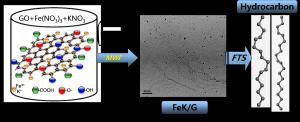
Recently, graphene as a high surface area support (2600 m2g-1, theoretical value) has shown unique properties and remarkable tunability in supporting a variety of metallic and bimetallic nanoparticle catalysts in heterogeneous catalysis. We discovered a new class of highly active Pd nanoparticle catalysts supported on graphene for carbon-carbon cross coupling reactions. These reactions are essential for the synthesis of large organic compounds for the pharmaceutical industry.
Very recently, we discovered a new class of highly efficient, selective and recyclable catalysts for the Fischer-Tropsch (FT) synthesis of long-chain hydrocarbons from syngas (H2 and CO). These catalysts consist of transition metal nanoparticles supported on the high surface area, thermally and chemically stable graphene. We are now developing several new FT catalysts supported on graphene for the selective synthesis of transportation fuels from syngas. The research also involves mechanistic studies focused on the role of graphene surface defects in the activity and selectivity of these catalysts. Several techniques such as high resolution microscopy (HRTEM, STEM and AFM) in addition to spectroscopic probes such as XPS, FT-IR and Raman spectroscopy are used to quantify the nature and extent of defects in the graphene support.
The students involved in this project will develop fundamentally new catalytic systems that will enable high efficiency hydrocarbon production with good selectivity and minimal byproducts. Controlled synthesis of size- and shape-selected metal nanoparticles combined with analytical tools and guided by theoretical calculations will enable the rational design and fundamental understanding of these catalytic systems.
Selected publications (nanomaterials, graphene and heterogeneous catalysis)
Co-Cu Bimetallic Metal Organic Framework Catalyst Outperforms the Pt/C Benchmark for Oxygen Reduction, M. Sanad, S. A. Puente, S. Tolba, Md A. Ahsan, O. Fernandez-Delgado, E. M. Hashem, M. S. Adly, M. A. Mahrous, M. S. El-Shall, S. Sreenivasan, N. Allam, L. Echegoyen, J. Am. Chem. Soc. 2021, 143, 4064-4073.
Efficient Removal of Heavy Metals from Polluted Water with High Selectivity for Hg(II) and Pb(II) by 2-imino-4-Thiobiuret Chemically Modified MIL-125 Metal-Organic Framework, M. S. Adly, S. M. El-Dafrawy, A. A. Ibrahim, S. A. El-Hakam, M. S. El-Shall, RSC Advances 2021, 11, 13940-13950.
High Performance Functionalized UiO Metal Organic Frameworks for the Efficient and Selective Adsorption of Pb(II) Ions in Concentrated Multi-ion Systems, G. S. Morcos, A. A. Ibrahim, M. H.
El-Sayed, M. S. El-Shall, Journal of Environmental Chemical Engineering 2021, 9, 105191.
Polyacrylonitrile Modified Partially Reduced Graphene Oxide Composites for the Extraction of Hg(II) Ions from Polluted Water, F. S. Awad, K. M. AbouZied, A. M. Bakry, W. M. Abou El-Maaty, A. M. El-Wakil, M. S. El-Shall, J. Mater. Sci. 2021, 56, 7982-7999.
Ligand‐Protected Ultrasmall Pd Nanoclusters Supported on Metal Oxide Surfaces for CO Oxidation. Does the Ligand Activate or Passivate the Pd Nanocatalyst?, M. Farrag, M. K. Das, M. Moody, M. S. El-Shall, ChemPhysChem. 2021, 22, 312-322.
Multifunctional Binding Sites on Nitrogen-doped Carboxylated Porous Carbon for Highly Efficient Adsorption of Pb(II), Hg(II) and Cr(VI) Ions, A. M. Bakry, F. S. Awad, J. A. Bobb, M. S. El-Shall,
ACS Omega 2020, 5, 33090-33100.
Physical and Chemical Synthesis of Au/CeO2 Nanoparticle Catalysts for Room Temperature CO Oxidation: A Comparative Study, K. Saoud, M. S. El-Shall, Catalysts 2020, 10, 1351.
Highly Fluorescent Hematoporphyrin Modified Graphene Oxide for Selective Detection of Copper Ions in Aqueous Solutions, F. S. Awad, K. M. AbouZied, A. M. Bakry, W. M. Abou El-Maaty, A. M. El-Wakil, M. S. El-Shall, Analytica Chimica Acta 2020, 1140, 111-121.
Melamine-based Functionalized Graphene Oxide and Zirconium Phosphate for High Performance Removal of Mercury and Lead Ions from Water, A. M. Bakry, F. S. Awad, J. A. Bobb, A. A. Ibrahim, M. S. El-Shall, RSC Advances 2020, 10, 37883-37897.
Heterogeneous Catalysis by Ultra-small Bimetallic Nanoparticles Surpassing Homogeneous Catalysis for Carbon-Carbon Bond Forming Reactions, N. Norouzi, M. K. Das, A. J. Richard, A. A. Ibrahim, H. M. El-Kaderi, M. S. El-Shall, Nanoscale 2020, 12, 19191-19202.
Laser-Assisted Synthesis of Gold–Graphene Oxide Nanocomposites: Effect of Pulse Duration, J. A. Bobb, C. J. Rodrigues, M. S. El-Shall, K. M. Tibbetts, Phys. Chem. Chem. Phys. 2020, 22, 18294-18303.
Green Synthesis of Oxide Supported Pd Nanocatalysts by Laser Methods for Room Temperature Carbon-Carbon Cross-Coupling Reactions, M. K. Das, J. A. Bobb, A. A. Ibrahim, A. Lin, K. M. AbouZeid, M. S. El-Shall, ACS Applied Mater. Interfaces 2020, 12, 23844-23852.
Preparation, Activity, and Mechanism of ZnIn2S4-based Catalysts for Photocatalytic Degradation of Atrazine in Aqueous Solution, L. Bo, H. D. Kiriarachchi, J. A. Bobb, A. A. Ibrahim, M. S. El-Shall, Journal of Water Process Engineering 2020, 36, 101334.
Enhancement of the Catalytic Activity of Pd Nanoparticles in Suzuki Coupling by Partial Functionalization of the Reduced Graphene Oxide Support with p-Phenylenediamine and Benzidine, A. A. Ibrahim, A. Lin, M. S. Adly, M. S. El-Shall, Journal of Catalysis 2020, 385, 194-203.
Laser Synthesis of Magnetite-Partially Reduced Graphene Oxide Nanocomposites for Arsenate Removal from Water, J. A. Bobb, F. S. Awad, S. Moussa, M. S. El-Shall, J. Mater. Sci. 2020, 55, 5351-5363.
Growth Mechanism of Sea Urchin ZnO Nanostructures in Aqueous Solutions and their Photocatalytic Activity for the Degradation of Organic Dyes, H. D. Kiriarachchi, K. M. Abouzeid, L. Bo and M. S. El-Shall, ACS Omega 2019, 14013-14020.
Pd-Fe3O4/RGO: A Highly Active and Magnetically Recyclable Catalyst for Suzuki Cross Coupling Reaction using a Microfluidic Flow Reactor, H. A. Elazab, A. R. Siamaki, B. F. Gupton, M. S.
El-Shall, Bulletin of Chemical Reaction Engineering & Catalysis 2019, 14, 478-489.
Nucleation and Growth of Gold Nanoparticles Initiated by Nanosecond and Femtosecond Laser Irradiation of Aqueous [AuCl4]-, C. J. Rodrigues, J. A. Bobb, M. G. John, S. P. Fisenko, M. S.
El-Shall, K. M. Tibbetts; Phys. Chem. Chem. Phys. 2018, 20, 28465-28475.
Plasmonic Chemically Modified Cotton Nanocomposite Fibers for Efficient Solar Water Desalination and Wastewater Treatment, H. D Kiriarachchi, F. S Awad, A. A Hassan, J. Bobb, A. Lin, M. S. El-Shall, Nanoscale 2018, 10, 18430-18539.
Laser Synthesis of Carbonaceous TiO2 from Metal–Organic Frameworks: Optimum Support for Pd Nanoparticles for C–C Cross-Coupling Reactions, J. A. Bobb, A. A. Ibrahim, M. S. El-Shall, ACS Appl. Nano Mater. 2018, 1, 4852-4862.
Plasmonic Graphene Polyurethane Nanocomposites for Efficient Solar Water Desalination, F. S. Awad, H. D. Kiriarachchi, K. M. AbouZeid, Ü. Özgür, M. S. El-Shall, ACS Applied Energy Materials 2018, 1, 976-985.
Tailoring the Reducibility and Catalytic Activity of CuO Nanoparticles for Low Temperature CO Oxidation, A. F. Zedan, A. T. Mohamed, M. S. El-Shall, S. Y. Al-Qaradawi, A. S. Al-Jaber, RSC Advance 2018, 8, 19499-19511.
Palladium Nanoparticles Supported on Ce-Metal Organic Framework for Efficient CO Oxidation and Low Temperature CO2 Capture, A. Lin, A. A. Ibrahim, P. Arab, H. M. El-Kaderi and M. S. El-Shall, ACS Applied Materials & Interfaces 2017, 9, 17961-17968.
Efficient Removal of Heavy Metals from Polluted Water with High Selectivity for Mercury(II) by 2-Imino-4-Thiobiuret Partially Reduced Graphene Oxide (IT-PRGO), F. Awad, K. M AbouZeid, W. M Abou El-Maaty, A. M El-Wakil, and M. S. El-Shall, ACS Applied Materials & Interfaces 2017, 9, 34230-34242.
Palladium Nanoparticles Supported on a Metal–Organic Framework-Partially Reduced Graphene Oxide Hybrid for the Catalytic Hydrodeoxygenation of Vanillin as a Model for Biofuel Upgrade Reactions, A. A. Ibrahim, A. Lin, F. Zhang, K. M. AbouZeid, ChemCatChem 2017, 9, 469–480.
The Effect of Graphene on Catalytic Performance of Palladium Nanoparticles Decorated with Fe3O4, Co3O4, and Ni(OH)2: Potential Efficient Catalysts used for Suzuki Cross Coupling Reactions, H. A. Elazab, S. Moussa, A. R. Siamaki, B. F. Gupton, and M. S. El-Shall, Catalysis Letters 2017, 147, 1510-1522.
Graphene Oxide Interface Enhances the Photochemical Synthesis, Stability and Photothermal Effect of Plasmonic Gold Nanostructures, H. S. Rady, A. N Emam, M. B Mohamed, and M. S.
El-Shall, Chem. Phys. Letters 2017, 690, 153-158.
Promotion Effect of Palladium on Co3O4 Incorporated within Mesoporous MCM-41 Silica for CO Oxidation, H. M.A. Hassan, M. A. Betiha, R. F.M. Elshaarawy and M. S. El-Shall, Applied Surface Science 2017, 402, 99–107.
Synergetic Catalysis of Palladium Nanoparticles Encaged within Amine-functionalized UiO-66 in the Hydrodeoxygenation of Vanillin in Water, F. Zhang, S. Zheng, Q. Xiao, Y. Zhong, W. Zhu, A. Lin and M. S. El-Shall, Green Chemistry 2016, 18, 2900-2908.
Water Inhibits CO Oxidation on Gold Cations in the Gas Phase. Structures and Binding Energies of the Sequential Addition of CO, H2O, O2 and N2 onto Au+, J. U. Reveles, K. M. Saoud and M. S. El-Shall, Phys. Chem. Chem. Phys. 2016, 18, 28606-28616.
Pt and Ni Nanoparticles Anchored into Metal–Organic Frameworks MIL-101(Cr) as Swift Catalysts for Ethanol Dehydration, H. M. Gobara, R. S. Mohamed, S. A. Hassan, F. H. Khalil and M. S. El-Shall, Catalysis Letters 2016, 146, 1875-1885.
Enhanced Photothermal Effect of Surface Oxidized Silicon Nanocrystals Anchored to Reduced Graphene Oxide Nanosheets, P. Afshani, S. Moussa, G. Atkinson, V. Y. Kisurin, and M. S.
El-Shall, Chem. Phys. Letters 2016, 650, 148-153.
Self-organization of Au–CdSe Hybrid Nanoflowers at Different Length Scales via Bi-functional Diamine Linkers, K. M. AbouZeid, M. B. Mohamed and M S. El-Shall, J. Nanoparticle Research 2016, 18, 1-13.
Palladium Nanoparticles Incorporated within Sulfonic Acid-Functionalized MIL–101(Cr) for Efficient Catalytic Conversion of Vanillin, Fumin Zhang, Yan Jin, Yanghe Fu, Yijun Zhong, Weidong Zhu, Amr Awad Ibrahim, and M. S. El-Shall, J. Mater. Chemistry A, 2015, 3, 17008-17015.
Highly Efficient and Magnetically Recyclable Graphene-Supported Pd/Fe3O4 Nanoparticle Catalysts for Suzuki and Heck Cross-Coupling Reactions, H. A. Elazab, A. R. Siamaki, S. Moussa, B. F. Gupton, and M. S. El-Shall, Applied Catalysis A 2015, 491, 58-69.
Reduced Graphene Oxide Doped with Ni/Pd Nanoparticles for Hydrogen Storage Application, Isamil, M. Madian and M. S. El-Shall, J. Industrial and Engineering Chemistry 2015, 30, 328-335.
Polyoxometalates Confined in the Mesoporous Cages of Metal–Organic Framework MIL-100 (Fe): Efficient Heterogeneous Catalysts for Esterification and Acetalization Reactions, F. Zhang, Y. Jin, J. Shi, Y. Zhong, W. Zhu, and M.S. El-Shall, Chemical Engineering Journal 2015, 269, 236-244.
Metal-Organic Frameworks with High Tungstophosphoric Acid Loading as Heterogeneous Acid Catalysts, A. S. Khder, H. M. A. Hassan and M. S. El-Shall, Applied Catalysis A 2014, 487, 110-118.
Direct Observation of Metal Nanoparticles as Heterogeneous Nuclei for the Condensation of Supersaturated Organic Vapors. Nucleation of Size-Selected Aluminum Nanoparticles in Acetonitrile and n-Hexane Vapors, V. Abdelsayed and M. S. El-Shall, J. Chem. Phys. 2014, 141, 054710.
P-Type Nitrogen-Doped ZnO Nanostructures with Controlled Shape and Doping Level by Facile Microwave Synthesis, N. P. Herring, L. S. Panchakarla and M. S. El-Shall, Langmuir 2014, 30, 2234-2240.
Graphene Supported Iron-based Nanoparticles for Catalytic Production of Liquid Hydrocarbons from Synthesis Gas. The Role of the Graphene Support in Comparison to Carbon Nanotubes, S. Moussa, L. Panchakarla, M. Ho and M. S. El-Shall, ACS Catalysis 2014, 4, 535-545.
Hydrogen Terminated Graphene by Laser Vaporization Controlled Condensation of Graphite Oxide. Observation of Hydrogen-Capped Carbon Chains CnH–, CnH+ and CnH2+ (n = 2-30) in the Gas phase, P. Afshani, I. Attah, S. Moussa, J. Terner, and M. S. El-Shall, J. Phys. Chem. C 2013, 117, 9485-9495.
Ultrasmall Gold Nanoparticles Anchored to Graphene and Enhanced Photothermal Effects by Laser Irradiation of Gold Nanostructures in Graphene Oxide Solutions, A. F. Zedan, S. Moussa, J. Terner, G. Atkinson, and M. S. El-Shall, ACS Nano 2013, 7, 627-636.
Rapid Synthesis of Magnetic/Luminescent (Fe3O4/CdSe) Nanocomposites by Microwave Irradiation, A. F. Zedan, V. Abdelsayed, M. B. Mohamed and M. S. El-Shall, J. Nanoparticle Res. 2013, 15, 1312-1318.
Laser Assisted Synthesis of Magnetic Fe/Fe2O3 Core-Shell Nanoparticles in Organic Solvents, S. Moussa, G. Atkinson, and M. S. El-Shall, J. Nanoparticle Res. 2013, 15, 1470-1479.
Pd-Partially Reduced Graphene Oxide Catalysts (Pd/PRGO): Laser Synthesis of Pd Nanoparticles Supported on PRGO Nanosheets for Carbon−Carbon Cross Coupling Reactions, S. Moussa, A. R. Siamaki, B. F. Gupton, and M. S. El-Shall, ACS Catalysis 2012, 2, 145−154.
Highly Efficient Electron Field Emission from Graphene Oxide Nanosheets Supported on Nickel Nanotip Pillar Arrays, D. Ye,S. Moussa,J. D. Ferguson,A. A. Baski and M. S. El-Shall, NANO Letters 2012, 12, 1265-1268.
Acid Catalyzed Organic Transformations by Heteropoly Tungstophosphoric Acid Supported on MCM-41, A. S. Khder, H. M. A. Hassan and M. S. El-Shall, Applied Catalysis A 2012, 411, 77-86.
Enhanced Photocatalytic Activity of ZnO–Graphene Nanocomposites Prepared by Microwave Synthesis, N. P. Herring, S. H. Almahoudi, C. R. Olson and M. S. El-Shall, J. Nanoparticle Res. 2012, 14, 1277-1289.
Microwave-Assisted Synthesis of Palladium Nanoparticles Supported on Graphene: A Highly Active and Recyclable Catalyst for Carbon-Carbon Cross-Coupling Reactions, A. R. Siamaki, A S. Khder, V. Abdelsayed, M. S. El-Shall and B. F. Gupton, J. Catalysis 2011, 279, 1-11.
Hybrid Au-CdSe and Ag-CdSe Nanoflowers and Core-Shell Nanocrystals via “One-Pot” Heterogeneous Nucleation and Growth, K. M. AbouZeid, M. B. Mohamedand M. S. El-Shall, Small 2011, 7, 3299-3307.
Laser Assisted Photocatalytic Reduction of Metal Ions by Graphene Oxide, S. Moussa, G. Atkinson,M. S. El-Shall, A. Shehata, K. M. AbouZeid and M. B. Mohamed, J. Mater. Chem. 2011, 21, 9608-9619.
Formation Mechanisms of Gold-Zinc Oxide Hexagonal Nanopyramids by Heterogeneous Nucleation using Microwave Synthesis, N. P. Herring, K. AbouZeid, M. B. Mohamed, J. Pinsk, and M. S. El-Shall. Langmuir 2011, 27, 15146-15154 (Journal Cover Feature).
Photothermal Deoxygenation of Graphite Oxide with Laser Excitation in Solution and Graphene-Aided Increase in Water Temperature, V. Abdelsayed, S. Moussa, H. M. Hassan, H. S. Aluri, M. M. Collinson, and M. S. El-Shall, J. Phys. Chem. Lett. 2010, 1, 204.
Ligand-Controlled Microwave Synthesis of Cubic and Hexagonal CdSe Nanocrystals Supported on Graphene. Photoluminescence Quenching by Graphene, A. F. Zedan, S. Sappal, S. Moussa and M. S. El-Shall, J. Phys. Chem. C. 2010, 114, 19920-19927.
Growth Mechanism of Anisotropic Gold Nanocrystals via Microwave Synthesis: Formation of Dioleamide by Gold nanocatalysis, M. B. Mohamed, K. M. AbouZeid, V. Abdelsayed, A. A. Aljarash and M. S. El-Shall, ACS Nano 2010, 4, 2766.